determination of sulphate in water by gravimetric method|gravimetric determination of sulfate : specialty store The analysis method was based on a suppressed conductivity system that simultaneously analyzed the anionic macroelements on Metrohm Supp A 250/4.0 column using 8.0 mM sodium carbonate (Na2. Whoops! Our server is Scratch'ing its head. We couldn't find the page you're looking for. Check to make sure you've typed the URL correctly.
{plog:ftitle_list}
Maria Hayashi's big titties bounce like wrecking balls as she gets banged on a balcony. Published by 41Ticket. 8 years ago . Related Videos. 05:14. Maria Hayashi's Wrecking Balls (Uncensored JAV) 230.6K views. 15:43. Sexy Japanese slut takes care of three cocks. Nippon Gangbang .
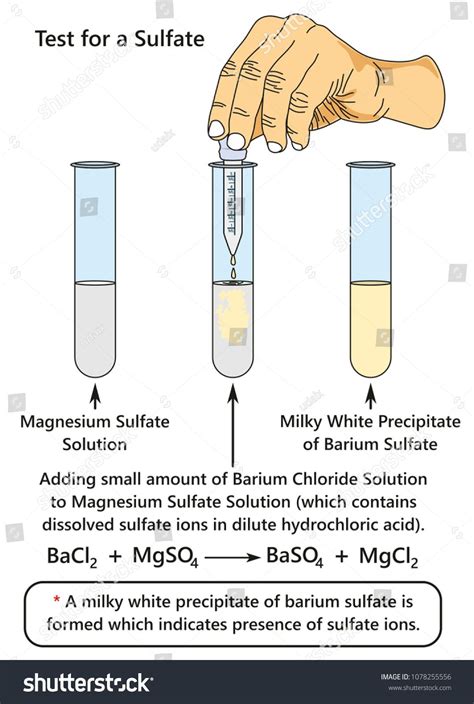
From the weight of the sample and weight of the precipitate, the percentage of sulfate in the sample is calculated. The precipitation reaction is the following: \ [Ba^ {2+} (aq) + SO_4^ {2-} (aq) \rightarrow BaSO_4 (s)\]In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will . The analysis method was based on a suppressed conductivity system that simultaneously analyzed the anionic macroelements on Metrohm Supp A 250/4.0 column using 8.0 mM sodium carbonate (Na2.1. Gravimetric Method with Ignition of Residue. Principle. Sulphate is precipitated in hydrochloric acid medium as barium sulphates by the addition of barium chloride.
In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will be determined by gravimetric analysis. First, a pre-weighed sample of the unknown . This document describes a procedure for determining the amount of sulfate in an unknown sample using gravimetric analysis with barium sulfate precipitation. Key steps include: 1) Adding barium chloride to the sample to .
The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION Gravimetric analysis is based on the .Gravimetric determination is determined by precipitating the unknown as an insoluble and then weighing the dried precipitate. To attain a good sample .Gravimetric Determination of Soluble Sulfate. The quantitative determination of sulfate ions in inorganic compounds can be accomplished by using the selective precipitation of the sulfate .Sulphate Gravimetric Method for Sulphate Determination: Theory: Nearly all sulphates in water can be precipitated as BaSO 4 on reaction with barium chloride (BaCl 2) under acidic .
GRAVIMETRIC DETERMINATION OF SULPHATE Irfan Ananda Ismail1, . A gravimetric method for analysis is usually based on a chemical . by evaporating the solvent or water that is still in the sample . 2. DETERMINATION OF SULPHATE AS BARIUMSULPHATE USING GRAVIMETRY WITH DRYING OF RESIDUE AIMS AND OBJECTIVES: To determine the amount of sulphate in an unknown sulfate sample To .METHOD 375.2. DETERMINATION OF SULFATE BY AUTOMATED COLORIMETRY. Edited by James W. O'Dell Inorganic Chemistry Branch Chemistry Research Division. Revision 2.0 . Dilution Water: Add 0.75 mL of sulfate stock solution (Section 7.10) and three drops of Brij-35 (CASRN 9002-92-0) to 2 L of reagent water.Using a technique called digestion is used in the gravimetric analysis to determine the amount of sulfate ion, SO4 2 - in an unknown sample. Statistical methods will then be applied to the experimental results of the group and of the whole class respectively to determine the success of the experiment.
for the determination of sulfate. Which indication method is the most suitable depends above all on the sample matrix. Method 1: Precipitation as barium sulfate and back-titration of the Ba2+ excess with EGTA. The ion-selective calcium electrode is used as indicator electrode. Method 2: As in method 1, but with the electrode combination .
sulphate concentration in water
sulphate and sulphide in water
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte . The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H 2 C 2 O 4, . The calcium sulfate (CaSO 4) in the tube retains carbon .University of Texas Arlington (UTA) Method The University of Texas Arlington method formulated by Petry (38) is also based on gravimetric analysis and uses a dilution ratio of 1:10. Ten (10) grams of soil is dissolved in 100 mL of distilled water, and the solution is shaken for 30 minutes to disintegrate sulfate salts in soil matrix.

Principle: It gives the most accurate results and is the recommended procedure for sulphate concentrations above 10 mg/mL. The sulphate ions in the sample are precipitated by the addition of barium chloride solution to water sample acidified with .
What is Gravimetric Analysis? Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a known volume of water is filtered, the collected solids are weighed. The principle of Gravimetric Analysis:Sulfate (Gravimetric) Current Revision. Issued 1974; Editorial Revision 1978: Media. WATER Instrumentation. Gravimetry: Method Subcategory. Inorganic: Method Source . Methods for the Chemical Analysis of Water and Wastes (MCAWW) (EPA/600/4-79/020) Brief Method Summary. Sulfate is precipitated as barium sulfate in a hydrochloric acid medium by .Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed .
A gravimetric method in which the . loss of a volatile species . gives rise to the signal (remove the volatile species) Moisture: determination of water in food content by heat or thermal or chemical energy (heating) Particulate gravimetry. A gravimetric method in which the . mass of a particulate analyte . isFormal Report of the Quantitative Determination of Sulphate by Gravimetric Analysis (Experiment 4) . in barium sulphate, by the gravimetric method. (C) Theory . Add 50 mL of water into the beaker, and then add 5 drops of concentrated HCL. Heat to boiling.Methods using potentiometric titrations and turbidity measurements have been more successful, but may require precise and costly equipment, which can present a barrier to some student laboratories.9 Another titrimetric procedure used for sulfate determination is conductometric titration, also known as conductometry, one INTRODUCTION The sulfate .
For example, one standard gravimetric method for the determination of magnesium involves its precipitation as MgNH 4 PO 4 •6H 2 O. Unfortunately, this precipitate is difficult to dry at lower temperatures without .Water sulfate testing is an important step in ensuring the safety and quality of water. There are a variety of methods available for testing water sulfates, each with their own advantages and disadvantages. In this paper, we will delve into the most common and widely used methods for testing water sulfates, such as gravimetric analysis, ion chromatography, and .Learn about gravimetric analysis, a method to determine the amount of a substance by measuring its mass.
In this paper, a simple and effective spectrophotometric method is described for the determination of sulfate in mine water. When the SO4 2− reacts with barium chloranilate at pH 4.5 in aqueous .Sulfate in aqueous solutions may be determined by a gravimetric method in which sulfate is precipitated as barium sulfate; the method is suitable for sulfate . 3.2 Water Sulfate concentrations in rain in Canada ranged between 1.0 and 3.8 mg/litre in 1980 (Franklin et al., 1985). An annual mean value of about 6 mg/litre in precipitation overStandard Methods Committee of the American Public Health Association, American Water Works Association, and Water Environment Federation. 4500-so42− sulfate In: Standard Methods For the Examination of Water and Wastewater. Lipps WC, Baxter TE, Braun-Howland E, editors. Washington DC: APHA Press. DOI: 10.2105/SMWW.2882.098Sulphate Gravimetric Method for Sulphate Determination: Theory: Nearly all sulphates in water can be precipitated as BaSO 4 on reaction with barium chloride (BaCl 2) under acidic conditions. Since this compound has very low solubility product, the precipitated BaSO 4 can be filtered and the residue weighed.
Sulfate concentrations are determined in mine water by gravimetric, titrimetric, colorimetric, turbidometric, ion chromatographic, inductively coupled plasma absorption spectrophotometric, and other methods. Accurate sulfate measurement of mine water can be difficult due to interfering groups, cations, and anions, mainly arsenate (AsO4 3−) and .
Gravimetric Determination of Chloride Introduction The chloride content of a soluble salt, or of an aqueous solution, can be . 3. Wash 3 beakers (600 mL) using soap and water, and rinse them with 3 portions of deionized water (25 mL portions). Clearly label these beakers so that you can tell them apart! 4. Using the analytical balance, weigh .Thermo gravimetric analysis method of analysis includes quantitative change in mass of analyte with reference to temperature. . the addition of adding barium chloride Barium sulphate has solubility about 3 mg L-1 at the ordinary temperature in water. The solubility of barium sulphate increases in presence of mineral acids due to formation of .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight of .
1. drying the sample 2. sample preparation (if the sample is water soluble, then it is dissolved in some volume of deionized water. If the sample is not soluble in water, an acid is usually used to dissolve the sample) 3. Precipitation of the analyte (a reagent, BaSO4, is added to the solution to precipitate the analyte.
sulfate determination chemistry
misuratore di umidità luce e ph acidità tester istruzioni
WEBRenato Shippee se rende e entra para o OnlyFans - Jornal de Brasília. O criador da personagem Karen Kardasha afirma que, sem preconceito, irá utilizar a plataforma +18 .
determination of sulphate in water by gravimetric method|gravimetric determination of sulfate